
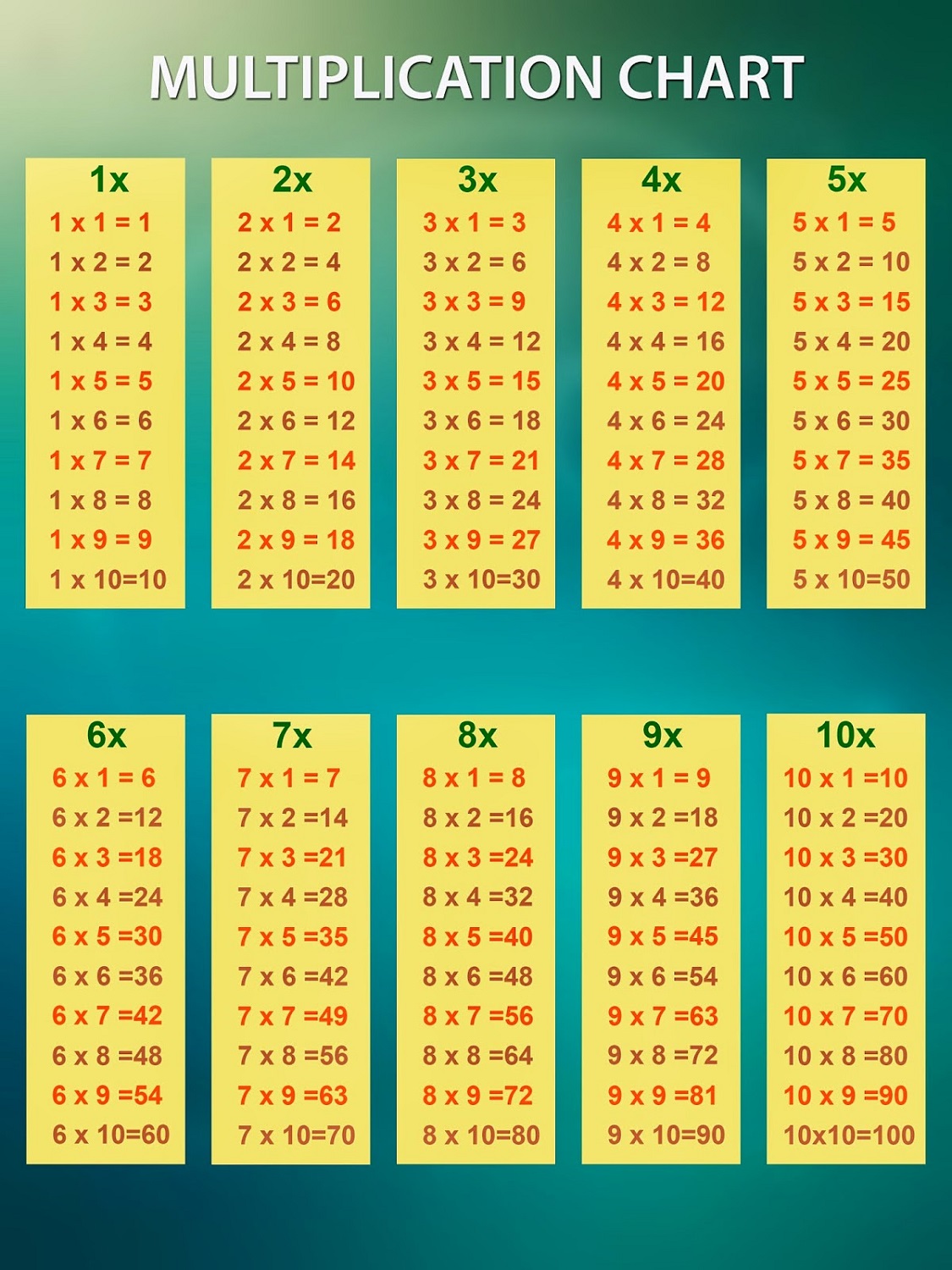


In HI assays, viruses with similar antigenic properties have antibody titer differences of less than or equal to 4-fold when compared to the reference (vaccine) virus. Ferret antisera are useful because antibodies raised against a particular virus can often recognize small changes in the surface proteins of other viruses.
Antigenic differences between viruses are determined by comparing how well the antibodies made against the vaccine reference viruses recognize the circulating viruses that have been grown in cell culture.
There are also prescription flu antiviral drugs that can be used to treat flu illness those need to be started as early as possible.ĬDC antigenically characterizes influenza viruses by hemagglutination inhibition (HI) (H1N1pdm09, B/Victoria, and B/Yamagata viruses) or neutralization-based HINT (H3N2 viruses) using antisera that ferrets make after being infected with reference viruses representing the 2022-2023 Northern Hemisphere recommended cell- or recombinant-based vaccine viruses. CDC continues to recommend that everyone ages 6 months and older get an annual flu vaccine as long as flu activity continues. All viruses collected and evaluated this season have been susceptible to the influenza antivirals peramivir, zanamivir, and baloxavir, and all viruses except for one (> 99.9%) have been susceptible to the influenza antiviral oseltamivir. The majority of influenza viruses tested are in the same genetic subclade as and antigenically similar to the influenza viruses included in this season’s influenza vaccine. CDC estimates that, so far this season, there have been at least 26 million illnesses, 290,000 hospitalizations, and 18,000 deaths from flu. Two influenza-associated pediatric deaths that occurred during the 2022-2023 season were reported this week, for a total of 117 pediatric flu deaths reported so far this season. Of the 49 influenza A viruses detected and subtyped during week 8, 63.3% were influenza A(H3N2) and 36.7% were influenza A(H1N1). The weekly rate of flu hospital admissions in the FluSurv-NET declined again during week 8. Hospitals reported 1,520 influenza hospitalizations to HHS Protect during week 8 compared to 1,817 reported during week 7. The number and weekly rate of flu hospital admissions decreased compared to week 7. Six of 10 HHS regions were below their outpatient respiratory illness baselines. Seasonal influenza activity remains low nationally.








 0 kommentar(er)
0 kommentar(er)
